Which of the Following Acids Ionizes Only Partially in Water
In an aqueous solution the H ion binds to a water molecule A water molecule that takes on a hydrogen ion is called a hydronium ion H3O. A substance present in smaller quantity is known as solute and a substance present in larger quantity is known as solvent.
16 1 Acid Ionization Ppt Download
Completely ionizes in water.

. An acid produces hydronium ions in water. A weak acid has a very low concentration. Many acids and bases are weak.
The spectator ions in the reaction between aqueous. The degree to which a weak acid ionizes. Acids that partially ionize in water are a.
Strong acids ionize completely when dissolved in water. The number of atoms. Hydrocyanic acid HCN ionizes partially in water according to the following equation.
An acid has a bitter taste. This problem has been solved. A weak acid is an acid that ionizes only slightly in an aqueous solution.
Hence it only ionizes partially into a solution. Weak and concentrated c. Hydrogen chloride HCl ionizes completely into hydrogen ions and chloride ions in water.
Pure water ionizes slightly or undergoes autoprotolysis. The electrical current generated in the solution is produced by. For sulfuric acid H2SO4 only the first hydrogen ionizes completely since its a diprotic acid.
A solution of a weak acid in water is a mixture of the nonionized acid hydronium ion and the conjugate base of the acid with the nonionized acid present in the greatest concentration. Weak acids ionize only partially in water. The metal forms anions.
2m b Calculate the pH of a 0030 M solution of hydrocyanic acid using the assumption made in a with and without. Hydrochloric acid is the stronger acid because it ionizes in water while acetic acid only slightly ionizes in water. Therefore the nature of CH 3 COO is basic and we call CH 3 COO the conjugate base of CH 3 COOH.
What acid ionizes only partially in water. Acids that partially ionize in water are a. For a list of the strong acids and bases try this.
Acetic acid is a weak acid and only ionizes about 1 into hydronium ions which makes less acidic higher pH. A weak acid ionizes only to a small extent in water. Hydrochloric acid is the stronger acid because it ionizes in water while acetic acid only slightly ionizes in water.
An equilibrium constant would have a value of infinity. See the answer See the answer See the answer done loading. Since there are different degrees of ionization there are different levels of weakness.
3An electrolyte is a substance that dissolves in water to produce a solution that conducts electricity. Thus a weak acid increases the hydronium ion concentration in an aqueous solution but not as much as the. Hydrogen gas is produced.
A strong acid will completely ionize in water while a weak acid will only partially ionize. A weak acid ionizes in water to produce hydronium ions. B Nitric acid is about 100 times more acidic than acetic acid because nitric acid solution contains about 100 times the number of hydronium ions.
HCN aq H 2 O l H 3 O aq CN - aq K a 501 x 10 -10 a What assumption can be made when calculating the pH of this solution. How can this acid solution best be described. When an acid reacts with an active metal a.
So when we add more and more of a solute into a solvent then there will occur an increase in the. An acid ionizes in water. Acid has a bitter taste.
NaOHs Naaq OHaq Substances that ionize completely which include strong acids and bases are called strong electrolytes and further include basically all water-soluble ionic compounds. An acid that only partially ionizes is called a _____- acid. Acetic acid is a weak acid which ionizes only partially in water a few percent.
Nitric _____ reacts with _____ to form copper nitrate nitrogen dioxide and water. Since a solution consists of solute and solvent. Hydrochloric acid is a strong acid and in water its ionization is complete.
An acid or bases strength refers to its degree of ionization. It is in equilibrium with its ions in water and its conjugate CH 3 COO a weak base is. Strong and dilute d.
One of the products of an acid reacting with a base. HCl is an acid according to the arrhenius definition because when placed in water it ionizes completely to form H ions and Cl- ions. Strong and concentrated b.
CH 3 COOH is a weak acid because it ionizes only partially in aqueous solution to form H and CH 3 COO ions. Which one of the following statements regarding a weak acid is NOT correct. A solution contains a large quantity of acetic acid dissolved in water.
Acetic acid is an example. Does a strong base have the same strength as a weak acid. Acetic acid only partially ionizes in water.
Because HCl is a strong acid its conjugate base Cl is extremely weak. A weak acid neutralizes bases. It is known that acetic acid is a weak acid.
A strong acid is an acid which is completely ionized in an aqueous solution. Carbon dioxide gas is produced. That is they do not ionize fully in aqueous solution.
CH 3 COOH CH 3 COO H. Why is this assumption valid. The hydronium ions concentration increases.

Strength Of Acids Boundless Chemistry
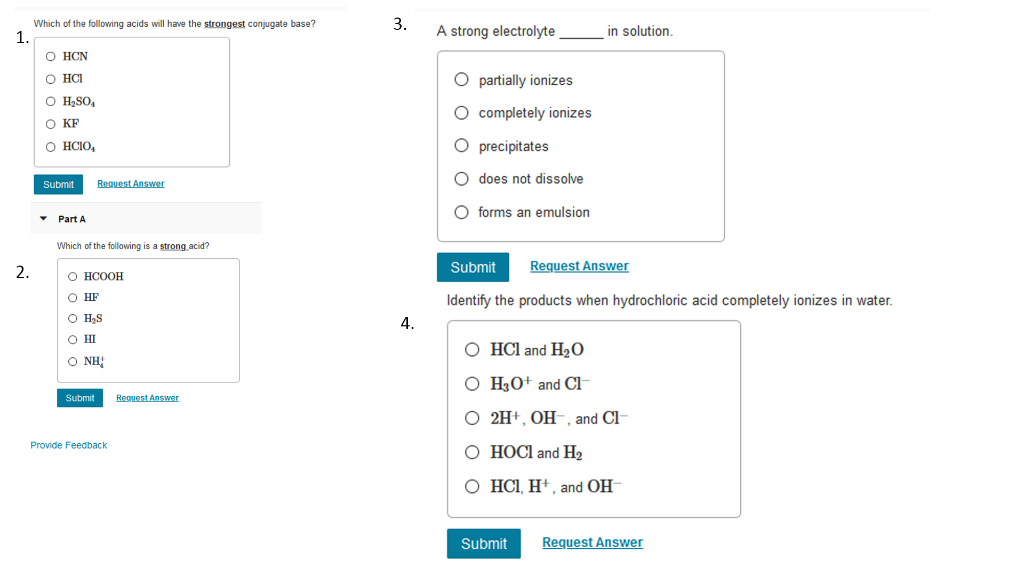
Solved Which Of The Following Acids Will Have The Strongest Chegg Com

Acid Base Strengths And Dissociation Constants Chemistry Jove

Solved In Water Substance That Ionizes Only Partially In A Solution Is Called Nonelectrolyte Weak Electrolyte Strong Electrolyte None Of These Is Correct Nonconductor

Analytical Chemistry Acid Base Arrhenius Theory H And Oh This Theory States That An Acid Is Any Substance That Ionizes Partially Or Completely In Ppt Download

Lesson Explainer Acidity And Basicity Nagwa

Flashcards P G 565 Flashcards Quizlet

Solved Acids That Partially Ionize In Water Are A Strong Chegg Com

Flashcards P G 565 Flashcards Quizlet

Hi Ya Ll Hope You Had A Good Weekend That Monday Wasn T Too Cruel I Know Many Of You Have Just Started College Or College Notes Study Notes Physics Notes
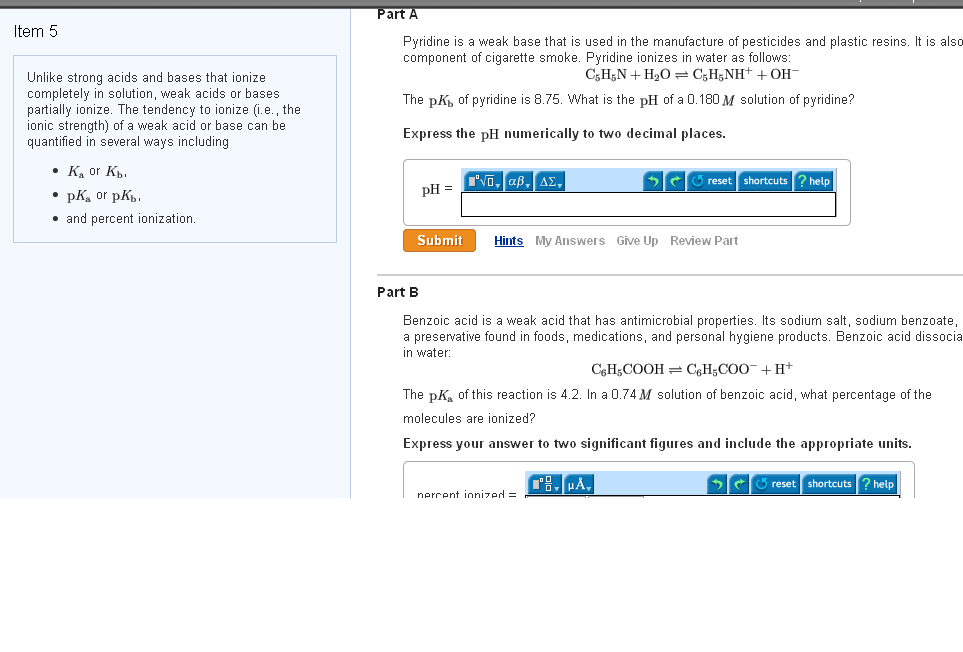
Solved Unlike Strong Acids And Bases That Ionize Completely Chegg Com

Question Video Recognizing The Difference Between Strong And Weak Acids Nagwa

Which Of The Following Correctly Describes A Weak Acid O It Completely Ionizes 0 It Produces Brainly Com

Strengths Of Acids And Bases Ppt Download
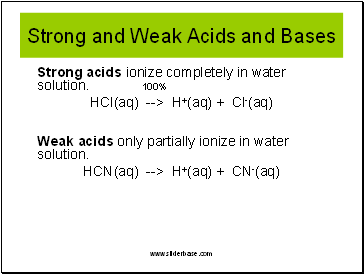
Acids And Bases Experimental Definitions Presentation Chemistry
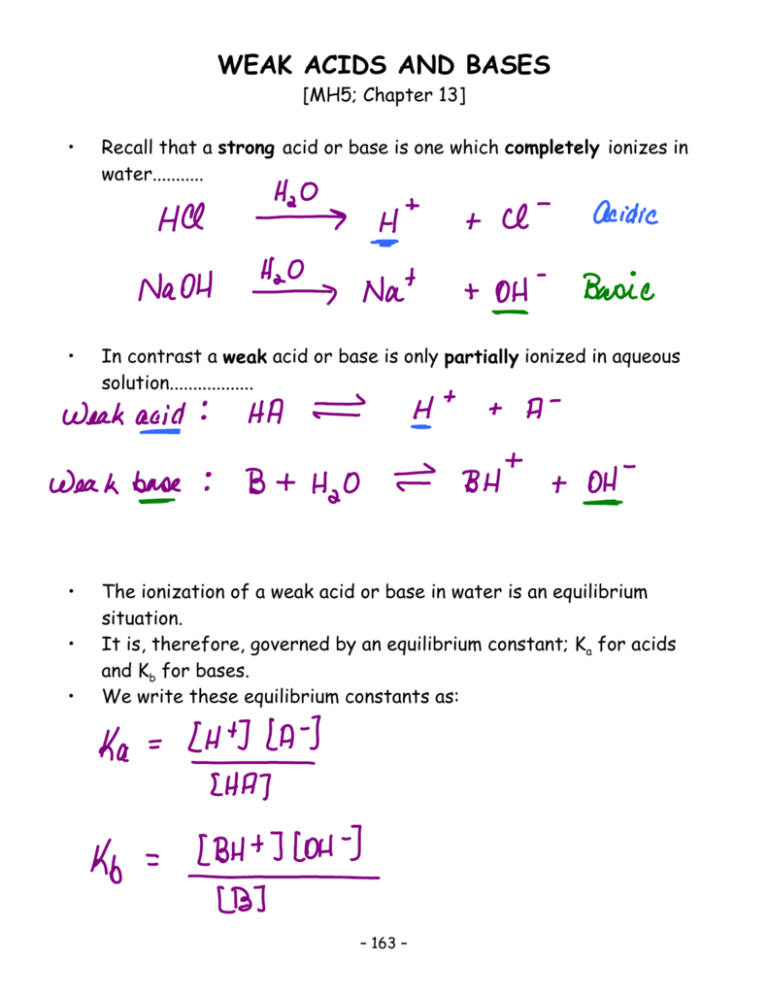
Completed Notes For Weak Acids And Bases
Solved Question 1 Which Of The Following Ionizes Partially Chegg Com


Comments
Post a Comment